VIRAL HEPATITIS
FECAL-BORNE HEPATITIS
Viral hepatitis is transmitted by food or water contaminated by fecal material. It is a serious inflammatory disease of the liver that is associated with poor sanitation and is common in developing countries. Two different viruses are commonly associated with fecal-borne hepatitis: hepatitis A virus (HAV) and hepatitis E virus (HEV).
Etiology
HAV is common in the U.S., whereas HEV is usually acquired by travelers to countries outside the U.S. Both viruses are single-stranded RNA non-enveloped viruses.
Manifestations
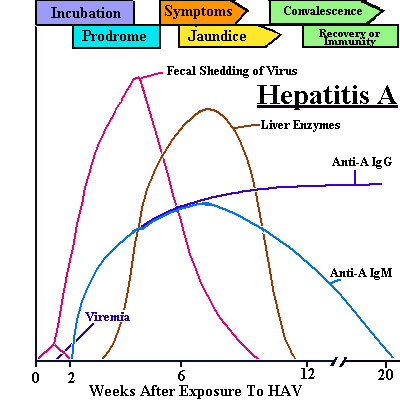
HAV infections have an incubation period of 14–45 days, with an average of 28 days. During this time, some patients who smoke may develop distaste for cigarettes. Most children with HAV infections are asymptomatic, whereas only a small number of adults are asymptomatic. Most symptomatic patients with HAV infection have jaundice.
The initial symptoms of HAV include fever (about 39°C), malaise, fatigue, headache, anorexia, nausea and vomiting, pain in the right upper quadrant, and hepatosplenomegaly. Classic symptoms of hepatitis may develop later; symptoms include cholestasis (dark urine and clay-colored stools) followed within 1–5 days by clinical jaundice (yellowing of skin and whites of eyes). The liver is enlarged and tender. Liver damage causes increased blood levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin. Groups or settings where people are at higher risk of acquiring an HAV infection are listed in Table HEP-1.
Table HEP-1. Groups at High Risk of Acquiring Hepatitis A Viral (HAV) Infection
Group
Comments
Settings
Comments
Travelers to countries with high rates of HAV infection
HAV remains one of the most common vaccine-preventable diseases of travelers
Food-service establishments
8% of food handlers have HAV
Men who have sexual activity with men (MSM)
HAV-infected MSMs report more frequent oral-anal contact, longer duration of sexual activity, and more sex partners than MSMs without serologic evidence of infection
Child care centers
Many children shed virus and are asymptomatic while shedding
Illicit drug users (injection and noninjection drugs)
Usually involves users of methamphetamine
Health-care institutions
Epidemiology
- About 44% of cases of viral hepatitis are caused by HAV, 49% by hepatitis B (HBV), and 7% by hepatitis C (HCV).
- 3,000- 4,000 cases of HAV are reported each year. Incidence in 2011 was 0.45 case per 100,000. Vaccination for HAV has significantly reduced the number of cases of HAV in the U.S.
- Natural hosts for HAV and HEV are humans and lower primates.
- The highest rate of HAV infection is among young adults 25–39 years of age. The prevalence of prior HAV infection among the general population in the U.S. is estimated to be 29–33%.
- Routine vaccination of children began in 1999 and that has significantly decreased the number of children infected by HAV.
- HEV is not endemic in the U.S.; however, residents of the U.S. who acquire HEV usually have a history of foreign travel. HEV epidemics have been reported in India, Pakistan, Nepal, Burma, North Africa, and Mexico.
- The incidence of HAV and HEV infections is higher in persons living in crowded living conditions and in areas of low socioeconomic development. More than 90% of the population in developing countries has been infected with HAV, whereas less than 50% of the population in developed countries has been infected with HAV.
- A chronic carrier state does not occur in patients infected with HAV or HEV. These two types of hepatitis are maintained in a population by serial transmission from subclinically infected persons to susceptible persons.
- Persons infected with HAV shed virus up to 10 days before symptoms begin. About 90% of children and 25–50% of adults shed virus and are asymptomatic.
- Mortality rates are very low in cases of HAV infection (0.1–0.2%). The mortality rate of HEV infection is higher than HAV infection in the general population (1–2%), and is even higher in pregnant women (20%) who have been infected with HEV in the third trimester.
- Person-to-person transmission via the fecal-oral route is the primary means of HAV and HEV transmission. Transmission occurs most frequently among close contacts in households and extended family settings, and can be transmitted following ingestion of fecally contaminated water or food.
- HAV infection is rarely transmitted following transfusion of blood or blood products that were collected from donors during the viremic phase of HAV infection.
- Sporadic outbreaks of HAV infection occur following ingestion of shellfish harvested from fecally contaminated waters.
Pathogenesis
After ingestion, HAV and HEV can withstand the harsh conditions in the stomach and intestines. These viruses replicate in the oropharynx and epithelial lining of the intestines, where they initiate a transient viremia and infect the liver. HAV and HEV bind to and replicate primarily within liver parenchymal cells. The viruses are released into bile and eventually into stool. HAV and HEV may be shed in feces for 10 days before clinical symptoms appear.
Although most infections are subclinical, lymphoid infiltration, Kupffer cell proliferation, and necrosis of the parenchyma cells can occur. Antibody-antigen complexes and complement fixation contribute to inflammation and tissue damage. All HAV infections are acute and are self-limited by the production of immunoglobulin M (IgM) and IgG reactive with the virus. These anti-HAV antibodies result in long-lasting immunity to future HAV exposure. Mortality rates are very low following HAV infection (0.1–0.2%), although high mortality rates (20%) are observed in woman infected with HEV during the third trimester of pregnancy. High mortality rates (20%) are observed in woman infected with HEV during the third trimester of pregnancy.

See how the antibodies to HAV rise in relation to viral titers and patient symptoms.
Diagnosis
Diagnosis of viral hepatitis includes identifying clinical signs and symptoms, including malaise, liver enlargement, jaundice, and icterus. Blood studies reveal elevated liver enzyme levels (ALT and AST). Serologic testing aids in confirmation of the clinical diagnosis (Table HEP-2).
Table HEP-2. Serologic Tests for Hepatitis A Virus (HAV) |
|||
Stage of Hepatitis Infection |
Immunoglobulin M (IgM)
|
Immunoglobulin G (IgG)
|
|
Acute HAV infection |
+ |
- |
|
Previous HAV infection |
- |
+ |
|
Therapy and Prevention
Supportive care and rest are currently the only treatment for patients with viral hepatitis. Immunoglobulin (Ig) given within 2 weeks of infection lessens the severity of symptoms or prevents symptoms. Ig is of no value once symptoms have appeared.
In the U.S., immunization with the HAV vaccine is recommended for all children at 1 year of age. Vaccination of children 1 year of age and older and adolescents and adults at the age-appropriate dose is preferred for those who plan to travel repeatedly or reside for long periods in intermediate or high-risk areas. All HAV vaccines contain inactivated (killed) virus and should be given in two doses at least 6 months apart.
Ig is recommended for travelers younger than 1 year of age and for persons 1 year of age and older who need only short-term protection. The HAV vaccine or Ig is recommended for all susceptible persons traveling or working in countries with intermediate or high rates of HAV infection.