Previous Lecture | Syllabus | Next Lecture
MM
526-541 and 145-152
![]()

2. To provide insight into the uses and misuses of antiviral vaccines.
3. To develop the concept of viral-mediated interference with viral replication.
4. To relate the lysogenic state to diseases thought to be caused by "dormant," "latent" or "slow" viruses.
5. To define the rational use of antiviral agents.
6. To gain familiarity with the structure and function of antiviral drugs.
IFN-
a
= a - Interferon
(20 subtypes) - from many different cell types
IFN
- b = b
- Interferon (2 subtypes) - from fibroblasts, macrophages
IFN
-g
= g - Interferon
(3 subtypes) - from T-lymphocytes
b. Interferons are virus non-specific.
c. Interferons are induced by viruses, chemicals, some species of bacteria and some extracts of fungi.
d.
Although all animal cells appear able to produce one or more types of interferon,
cells of the bone marrow, spleen,
and macrophages, appear to play a special role, i.e., they produce a larger
volume of interferon and a more
potent interferon.
For most RNA viruses, double-stranded RNA segments produced during replication mediate the induction of interferon.
Interferons are synthesized on membrane-bound polysomes. As they are formed, they are segregated into vesicles and are glycosylated; from the vesicles they are excreted outside the cells. Therefore until they are excreted, interferons do not act on the cell that produces them.
Mechanism of interferon action

Suggested mechanisms for the antiviral action of interferon.
Interferons cause antiviral resistance not directly, but by activating cellular genes for antiviral proteins; they are ineffective in enucleated cytoplasts or in the presence of actinomycin D.
Interferons induce the cell response by interacting with the cell surface. At the cell surface, interferons bind to receptors containing gangliosides (glycosylated phospholipids); transformed cells, which are deficient in gangliosides, are less interferon-sensitive than normal cells. In human cells, genes specifying the receptors are present on chromatosome 21; 21-trisomic (Down's syndrome) cells are especially sensitive to interferon. The molecular mechanisms of interferon-induced anti-viral resistance are multiple, and probably differ in different cell-virus systems. However, in vitro studies with extracts of interferon-treated cells show that the main target of interferon action is translation, which is blocked by two mechanisms, involving a protein kinase and a nuclease. In both, the block requires the presence of minute amounts of double-stranded RNA (dsRNA), which seems to signal to the cells the presence of a viral infection. (As in the induction of interferon, the dsRNA may be that of a viral replicative intermediate, or it may result from symmetric transcription of the viral DNA). Both translation blocks are therefore specific for virus-infected cells (containing dsRNA), although they do not distinguish cellular and viral messengers within such cells.
Interferons exhibit a wide variety of cell regulatory activities. They can be considered a family of hormones involved in regulation of cell growth and differentiation. The cell regulatory activity of IFN - g is much greater than that of IFN - a or IFN - b . Effects of interferon on the functions of the uninfected cells:
b. Inhibition of activation of spleen lymphocytes;
c. Inhibition of liver regeneration;
d. Inhibition of production of platelets and leukocytes;
e. Enhancement of the expression of histocompatibility antigens on the lymphocyte surface;
f. Induction of liver degeneration;
g. Reduction of antiviral antibody production by inhibition of B-cell activity;
h. Enhancement of T-cell activity; and
i. Increase in the cytotoxic activity of natural killer cells against virus-infected cells.
Principal vaccines used in prevention of viral diseases of humans
|
|
|
|
|
|
|
| Poliomyelitis | Tissue culture (human diploid cell line, monkey kidney) | Live
attenuated
Killed |
Oral
Subcutaneous |
| Measles | Tissue culture (chick embryo) | Live attenuated | Subcutaneous |
| Mumps | Tissue culture (chick embryo) | Live attenuated | Subcutaneous |
| Rubella | Tissue culture (duck embryo, rabbit, or human diploid ) | Live attenuated | Subcutaneous |
| Hepatitis type B | Purified HBsAg from "healthy" carriers; HBsAg from recombinant DNA in yeast | Subunit | Subcutaneous |
| Chickenpox/Zoster | Tissue culture | Live attenuated | Subcutaneous |
|
|
|
|
Route of Administration |
| Smallpox | Lymph from calf or sheep
(glycerolated, lyophilized)
Chorioallantois, tissue cultures (lyophilized) |
Live vaccinia | Intradermal: multiple pressure, multiple puncture |
| Yellow Fever | Tissue cultures and eggs (17D strain) | Live attenuated | Subcutaneous or intradermal |
| Hepatitis type A | Tissue culture | Killed | Subcutaneous |
| Influenza | Highly purified or subunit forms of chick embryo allantoic fluid (formalinized or UV-irradiated) | Killed | Subcutaneous or intradermal |
| Rabies | Duck embryo or human diploid cells | Killed | Subcutaneous |
| Adenovirus | Human diploid cell cultures | Live attenuated | Oral, by enteric-coated capsule |
| Japanese B encephalitis | Mouse brain (formalinized), tissue culture | Killed | Subcutaneous |
| Venezuelan equine encephalomyelitis | Guinea pig heart cell culture | Live attenuated | Subcutaneous |
| Eastern equine encephalomyelitis | Chick embryo cell culture | Killed | Subcutaneous |
| Western equine encephalomyelitis | Chick embryo cell culture | Killed | Subcutaneous |
| Russian spring-summer encephalitis | Mouse brain (formalinized | Killed | Subcutaneous |
Inactivated virus vaccines - Inactivated vaccines generally stimulate the development of circulating antibody against the capsid or envelope proteins of the virus, conferring some degree of resistance. However, there are disadvantages to this type of vaccine:
b. The immunity conferred is often brief and directed against only external antigens (capsid, envelope, spike);
c.
Parenteral administration of inactivated vaccine sometimes gives limited
protection because local resistance
is not induced adequately at the natural port of entry or primary site
of multiplication of the wild virus
infection; and
d. Some inactivated virus vaccines induce hypersensitivity to subsequent infection or booster shots.
b. Unrecognized adventitious agents latently infecting the culture substrate may enter the vaccine stocks; and
c. Limited shelf life.
b.
A virus that is not pathogenic, e.g., the vaccinia virus, is altered by
the insertion of a gene from a pathogenic
virus. The inserted gene codes for protein (e.g., the capsid protein) which
elicits an immune reaction and
protects against the pathogenic virus.
DNA vaccines (gene vaccines). Viral DNA is inserted into human cells where it codes for proteins that are expressed on the surface of the human cell, thus stimulating the immune system.
C.
Lysogenization, latency, dormancy. Normally when a virus enters a cell,
it reproduces at a rapid rate and produces
large numbers of progeny virus. In rare cases, the virus enters the cell
and persists in the cell with little or no
detectable effect on the host cell. This is gusually called a latent or
dormant infection. These latent infections are
sometimes activated by systemic shock. The basis for this latency
has been attributed to:
2. Lack of pathogenicity of the virus; and
3. Suppression of reproduction by the immune system (humoral, cell mediated, interferon).
1. Acycloguanosine (Acyclovir) (Zovirax)
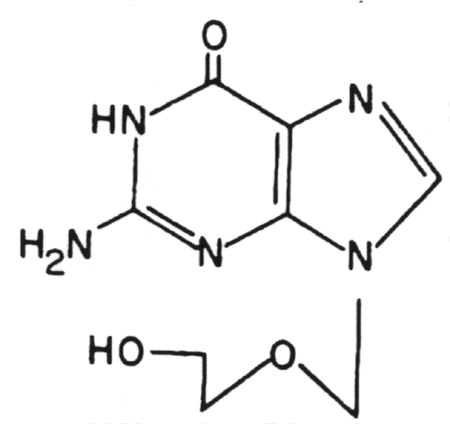
2.
Valacyclovir (Valtrex)
This is the L-valyl ester of acyclovir. It has the same spectrum of activity as acyclovir.
3. Adenine arabinoside (Ara A) (Vidarabine)
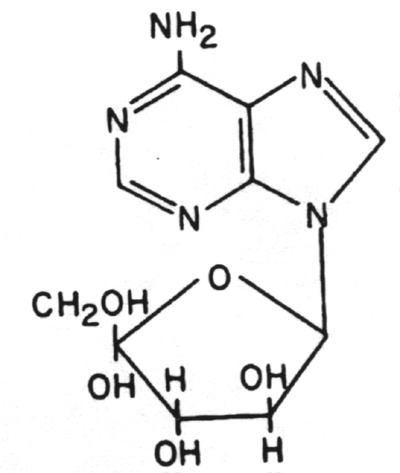
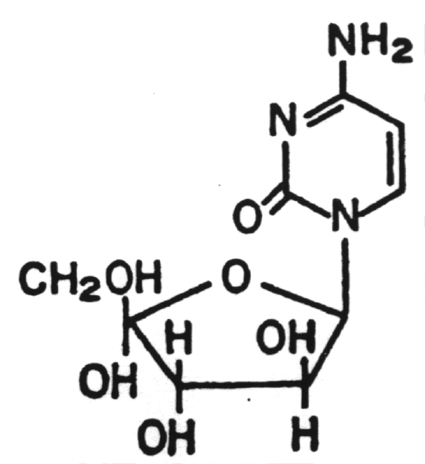
4. Cytosine arabinoside (Ara C)
Licensed only as an anti-tumor drug.
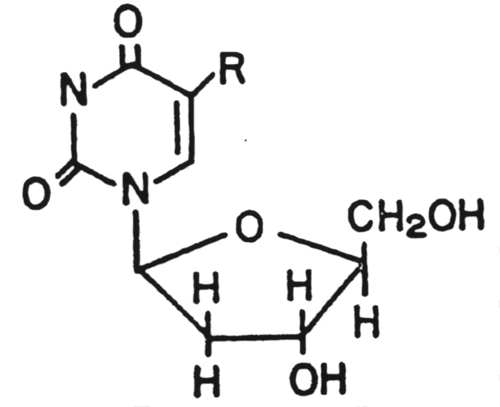

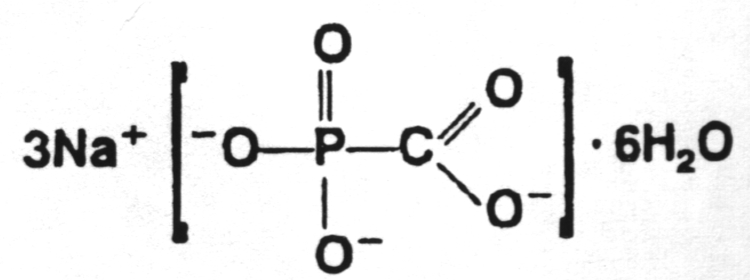
8.
Ganciclovir (Cytovene)

This drug was approved by the U.S. Food and Drug Administration in 1998 for the treatment of cytomegalovirus retinitis. It is unique in that it is the first "antisense" antiviral approved by the FDA. An "antisense" vaccine is one which is a single-stranded DNA complement to a mRNA. The DNA and mRNA bind to each other and prevent the translation process. It is administered directly into the eye.10. Amantadine
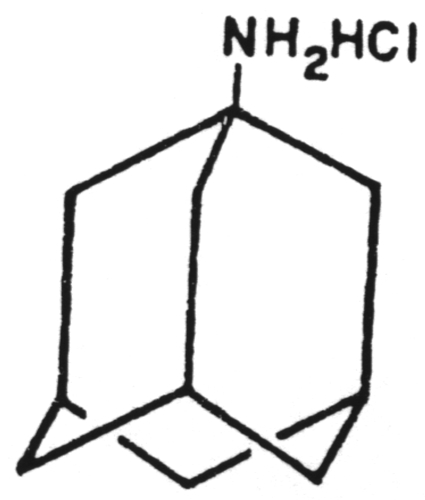
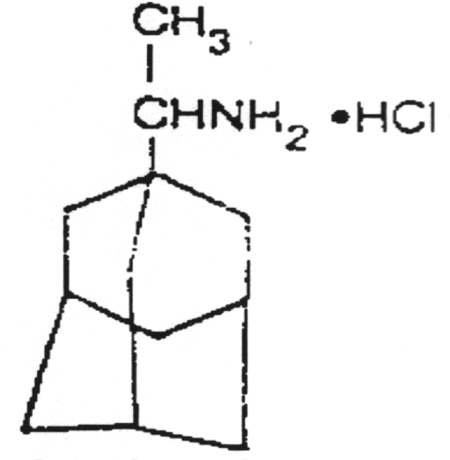
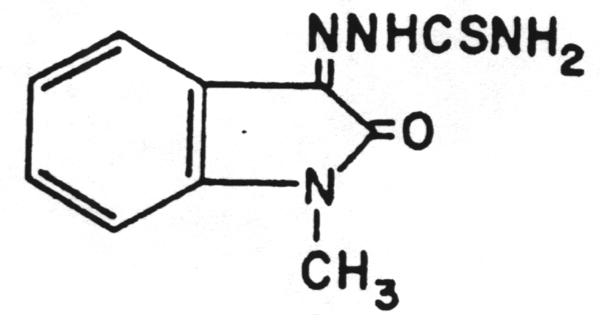
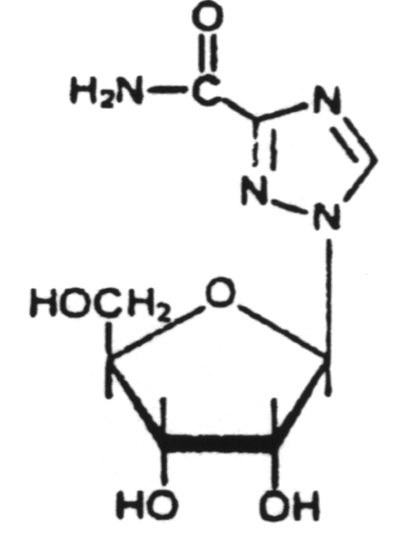
A chain terminator activated via phosphorylation by thymidine kinase.
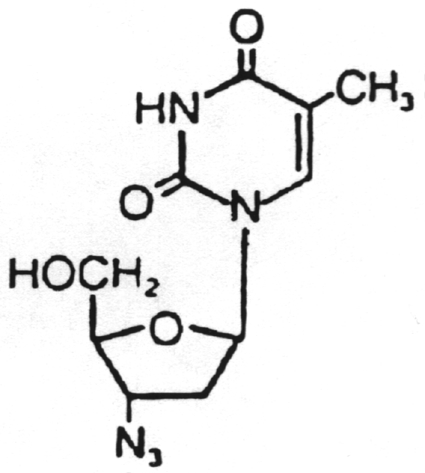
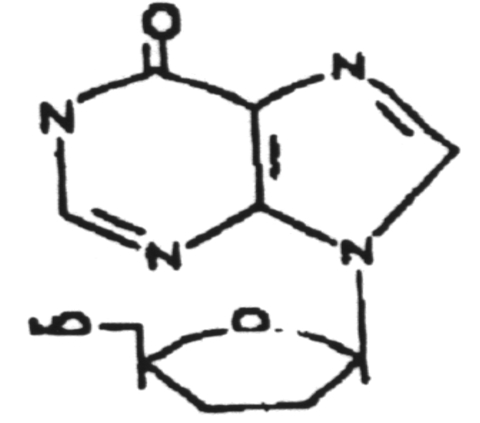
Dideoxyinosine
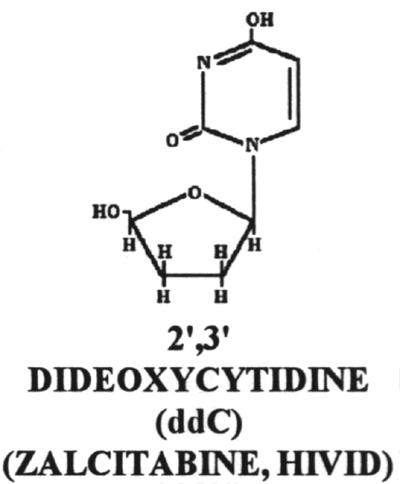
18. Dideoxycytidine (ddC)
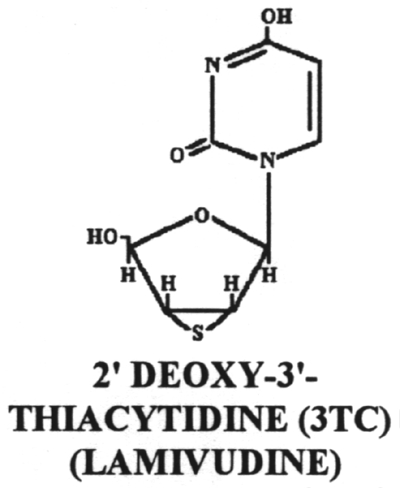
20.
Stavudine, d4T
This drug is used to treat AIDS, but it can only be used in combination with AZT and a protease inhibitor in patients who have never been treated previously with AZT. It is a nucleoside analog that inhibits reverse transcriptase.21. Delavirdine
This is a nonnucleoside inhibitor of reverse transcriptase.22. Nevirapine

This is a nonnucleoside inhibitor of reverse transcriptase.23. Saquinavir (Invirase) (Hoffman - LaRoche)
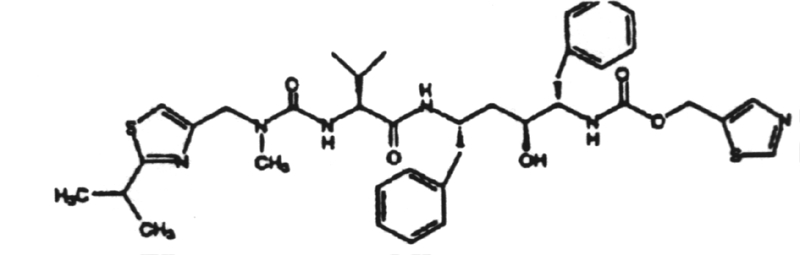
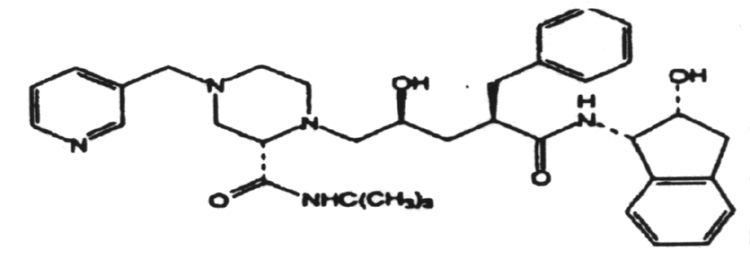

2. The induction of interferon is mediated by double-stranded RNA.
3.
In uninfected cells interferons act like a family of hormones involved
in regulation of cell growth and differentiation
where they often effect a shift from humoral to cell-mediated immunity.
4.
Effective vaccination for viral disease can be achieved using live viruses,
inactivated viruses, viral antigens or
antibody directed against viruses.
5.
Pediatric patients are routinely immunized against the viral diseases of
polio, measles (rubeola), mumps and
rubella. Several other viral vaccines are available for special situations.
6.
In general the live attenuated viral vaccines induce the broadest range
of immunity (i.e., stimulate IgA, IgG and IgM
production) and give the longest acting immunity.
7.
Some viruses have the ability to be dormant or latent in a cell during
which time they do not reproduce
themselves. Most commonly they do this by integrating their DNA into the
host cell DNA.
8.
The antiviral agents currently available are narrow spectrum compounds,
most of which are analogs of the purine
or pyrimidine bases of DNA or RNA.
9. The antiviral drugs and the diseases they are effective against are:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|